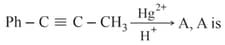Chemical Properties of Alkynes
Chemical Properties of Alkynes: Overview
This topic covers concepts such as Chemical Reactions of Alkynes, Acidic Character of Terminal Alkynes, Electrophilic Addition Reactions of Alkynes, Halogenation of Alkynes, Hydrohalogenation of Alkynes, Hydration of Alkynes, etc.
Important Questions on Chemical Properties of Alkynes
When propyne is treated with aqueous in presence of , the major product is:
The product(s) obtained via hydration of 1-butyne would be:
The number of possible structural and configurational isomers of a bromo compound, formed by the addition of 1 mole of HBr to 2-pentyne respectively, are –
The polymerization of acetylene with Ziegler–Natta catalysts produces polyacetylene films.
When acetylene, an alkyne passed through red-hot iron tube gives
Propyne on reaction with tollens reagent produces a white precipitate.
Write the chemical equation for the reaction of propyne with tollens reagent.
In presence of , the ethyne is first converted into vinyl acetylene which is the starting material of neoprene.
Thin films of polyacetylene can be used as electrodes in batteries.
The alkyne used in the preparation of chloroprene is _____.
The catalyst used in the polymerization of Alkynes is _____.
Write a note on linear polymerization of alkyne.
What would be the expected product of the reaction of propyne with if the mechanism of this reaction is analogous to that of propene?(Bromoacetone/Bromopropanol)
A hydrocarbon with molecular formula decolorizes bromine water and forms a white precipitate in ethanolic solution Treatment of with in aqueous produces a compound, which gives a yellow precipitate when treated with and . The structure of is :
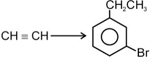
The following conversion can be carried out by
An organic compound X with molecular formula when treated with forms a gem dibromide. The compound X upon warming with and dil. produces a ketone, which gives a positive iodoform test. The compound X is
An alkyne forms sodium alkynide and when reacted with two molecules of , it gave What are and
Select reaction(s) which will favour in the forward direction: